In 2013, a group of scientists from British biotech company, Oxitec, embarked on a risky experiment in a remote region of Brazil.
They wanted to study the impact of releasing a new strain of mosquitoes into the wild.
Mosquitoes have been a terrible scourge in this region for decades. In warm and wet conditions, the insects had thrived: helping to spread diseases such as dengue, yellow fever and malaria.
And so Oxitec offered a novel solution…
Over 27 months of testing, they would release 450,000 male mosquitoes into the wild: each one genetically modified so that it couldn’t reproduce.
The idea was that these gene-hacked insects mixed with wild mosquitoes, the population would begin to drop off.
And for a while it worked beautifully.
The population of mosquitoes in Jacobino completely collapsed: almost 85% were wiped out.
But last week brought some disturbing news…
According to research published in Nature Scientific Reports, the population of mosquitoes in the region has recently bounced right back.
And even worse, a new genetic hybrid has emerged that is more resilient than the wild strain that they tried to wipe out.
“The claim was that genes from the release strain would not get into the general population because offspring would die,” said Yale researcher Jeffrey Powell, one of the researchers behind the new paper.
“That obviously was not what happened.”
Nature bites back
This study highlights some of the problems we’ll face with gene editing.
We can use extraordinary new tools – such as CRISPR – to change the genes of species, diseases and plant life.
And these can work beautifully in the lab.
But when we release them into the wild it can be a bit of a lottery.
Nature finds a way to bite back.
In this case, it seems females of the native species of mosquito began to discriminate: avoiding mating with modified males.
Soon enough, a new population of hybrids was thriving.
And as we look to edit the genes of animals, plants and even human embryos, we will see more cases like this.
How to profit from safe gene-editing
That’s why my research on gene editing has been focused on three areas.
These are fields with a long history of gene editing.
And after decades of trial and error, companies in these fields are already making huge progress in successfully introducing their gene-edited organisms into the local population.
Take the example of animal genetics company Genus (LON: GNS).
1. Cows and Swine
Genus analyses the DNA of cows and pigs to select the healthiest parents and herds for farmers.
The breeding selection programme tests approximately 350 dairy bulls per year in a rolling five-year programme which includes creating and measuring the output of 35,000 daughters.
Their first big breakthrough came in 2015 when they bred pigs resistant to Porcine Reproductive and Respiratory Syndrome known as “blue ear” disease because of the bluish discoloration to the ears and body that infected pigs display.
This is a disease that has been ruining the swine industry.
It attacks immune cells in the lungs causing severe breathing problems in young pigs and reproductive failure in pregnant females.
Blue ear is endemic in most pig producing countries.
It has persisted in China at low levels since a major outbreak in 2006 killed some 400,000 pigs and affected 2 million more.
In Europe alone the disease is estimated cost the pig industry more than 1.5 billion euro each year.
By using gene-editing technology scientists were able to target and remove a specific gene – the CD163 gene – that’s vital in the development of blue ear disease.
They were then able to create four distinct “elite populations” of male and female pigs that have been genetically fortified to resist Blue Ear.
And since then they’ve been working with the US Food and Drug Administration to obtain regulatory approval to commercialise the pigs in America.
It’s a delicate issue.
This gene editing technology has huge potential and allows
scientists to make changes to an animal’s genome that might otherwise take years of selective breeding.
The most common technique known as CRISPR-Cas9 acts as ‘molecular scissors’ which target a specific point on the genome and cut it open.
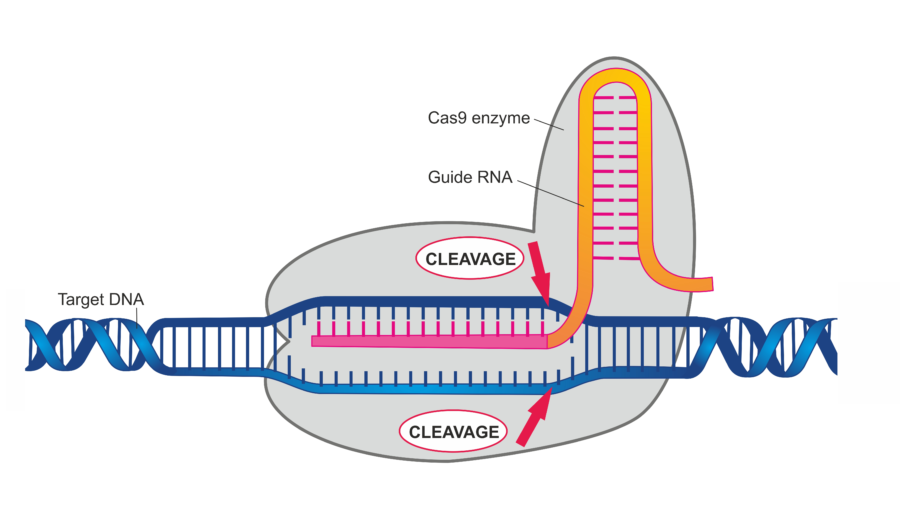
The cells’ DNA repairs the break and introduce changes to one or more gene enabling scientists to precisely remove add or alter sections of the DNA sequence.
But CRISPR is obviously also a controversial technology.
And it will take time to achieve regulatory approval for any breakthroughs in makes in gene editing.
2. Survivor Crops
Agriculture is another area with a long history of gene editing. Crop scientists have been studying the impact of cross breeding for decades.
Norman Borlaug (pictured), the father of crop science, was a keen advocate. He spent ten years stooped in baking Mexican grain fields to develop a strain of wheat that, when properly charged with water and fertiliser, could triple crop yields.

In the fifties, Borlaug’s short-and strong-stemmed wheat helped Mexico double production. His wheat then swept across India, raising yields from 12 million tonnes in 1965 to 20 million by 1970, and kick started an agricultural revolution.
But there are serious limits to this type of farming. It made farmers in much of the world dependent on fertiliser, pesticides and costly equipment.
Borlaug had a solution – a ‘gene revolution’. He recognised that GM seeds could achieve in weeks what had taken him a decade of hard toil.
And today CRISPR technology helps producers optimise photosynthesis and the vitamin content of crops.
Since it was first tested on tobacco production in 2013, CRISPR has been used on a range of crops, from wheat and rice to oranges and tomatoes; and for a whole spectrum of applications — from boosting crop resistance to pests, to improving nutritional contents.
Indigo Agtech – recently voted the most disruptive company in the world by CBNC – specialises in making “survivor crops”:
These include crops that are resistant to drought.
Indigo uses naturally occurring microbes instead of genetic modifications or chemicals to help plants survive and grow in rough conditions.
Indigo Agriculture’s beneficial microbes can be applied as a liquid or powder coating to seeds (a standard process in agriculture already).
They also can be sprayed onto flowering plants, which take the beneficial microbes into their seeds.
In 2016 and 2017, Indigo found its microbes resulted in an 11% yield improvement and a 14% yield improvement on cotton farms in drought-stricken Texas.
Indigo also collects a huge amount of data on crops and farming practices: using sensors, drones and satellites to send one trillion data points per day back to Indigo’s Boston headquarters for analysis.
It’s one huge agriculture lab: drawing information from 125 farm businesses averaging 8,000 acres in size, spread across the world.
You can read more about the business here.
(An IPO may not be far away).
Meanwhile, Syngenta sees CRISPR-modified corn as a big opportunity in China, which grows more hectares of corn than any other crop.
According to Science Magazine…
“Yields per hectare are only 60% of those in the United States because corn ear worms often weaken Chinese crops.
A fungus thrives in the weakened plants, producing a toxin that makes the resultant ears unfit for animal feed.
As a result, China must import a great deal of corn. (According to USDA, 82% of U.S.-grown corn has been engineered to have a bacterial gene that makes it resistant to ear worms.)
CRISPR could allow Syngenta to quickly modify the corn genomes to introduce insect resistance or other traits, bolstering China’s food supply while transforming agribusiness there.”
3. Biofuels
We are also learning to safely tweak plants to create biofuels.
Synthetic Genomics has been working with Exxon on scaling up the use of algae as a serious biofuel contender to fossil fuels.
The Synthetic Genomics team, led by Craig Venter, identified the genetic switch for producing oil in the algae species Nannochloropsis gaditana, then tweaked it to produce oil even when nitrogen is plentiful.
The result, was a doubling of the algae’s oil content – from 20% to more than 40% – with no significant impact on the algae’s growth.
Exxon says that by 2025 it will be producing 10,000 barrels a day of this biofuel.
Algae is hugely promising as a biofuel: appearing green in colour due to the chlorophyll—a light-capturing pigment—inside them, microalgae carry out photosynthesis as plants do, capturing the sun’s energy and absorbing carbon dioxide to grow.

Algae grow in salt water so don’t affect the supply of agricultural land and suck up Co2 as they grow.
The difficulty with scaling algae production is that it adapts to its environment.
We may find an echo of the mosquito problem: what works in the lab may not work in practice.
Bigger volumes lead to higher microalgal concentrations, which are difficult to control as the algae adapt to shading conditions and the release of carbon dioxide.
Euglena (Tokyo: 2391) is a company in this field that I follow closely (though they are not a gene-editing company).
Researchers at Euglena found ways to hijack microalgae into biofuel factories. As Euglena scientists recently told Asian Scientist Magazine, the trick is to tweak the conditions under which microalgae are cultivated…
“Some types of microalgae belonging to the genus Euglena can be efficiently grown under conditions of high carbon dioxide.
Basically, we introduce carbon dioxide and light into a culture device containing a fixed number of microalgae cells, allowing those cells to photosynthesize and grow.
Thereafter, the cells are recovered and kept in a dark, oxygen deficient state. Tricked into thinking that they need to endure a period of starvation, the cells begin to store wax ester, a feedstock for biodiesel and aviation biofuel.
The microalgae are subsequently dried, and the wax ester is extracted using an organic solvent. In this manner, microalgae double up as a carbon sink and biofuel source.”
Euglena started building its first commercial-scale algae biodiesel production facility last year with a production capacity of 125,000 litres per year.
According to the Japanese government-led Green-oil Japan project, biofuel usage in the country will increase from 125 kiloliters in 2018 to 1 million kiloleters by 2030.
And Euglena will be central to that plan.
It’s currently working on the production of biofuel for jet planes that will be used in the Tokyo Olympics next year.
A company that is well worth a look.
I’ll update on the most promising investments here in MD as we go.

